30 januari 2018
Philips’ resultaten vierde kwartaal en jaarresultaat 2017
Philips behaalt in het vierde kwartaal een omzet van EUR 5,3 miljard, met een groei van 5% op vergelijkbare basis; het nettoresultaat uit doorlopende activiteiten bedraagt EUR 476 miljoen, en de gecorrigeerde EBITA is toegenomen met 140 basispunten naar 16,7 procent
Kerncijfers vierde kwartaal Kerncijfers hele jaar Frans van Houten, CEO: Uit de resultaten van Philips in het vierde kwartaal blijkt dat we aan kracht winnen. We hebben 2017 sterk afgesloten met een vergelijkbare groei van de orderontvangst van 7% en een vergelijkbare omzetgroei van 5%, met name dankzij de activiteiten Personal Health en Diagnosis & Treatment. De gecorrigeerde EBITA-marge is sterk verbeterd met 140 basispunten. Dit is voornamelijk het gevolg van hogere volumes, inkoop- en productiviteitsbesparingen, en de stijging van de vrije kasstroom naar EUR 948 miljoen. We hebben ons portfolio verder versterkt met een aantal gerichte acquisities verspreid over het zorgcontinuüm. De integratie van deze acquisities ligt op schema. Ik wijs er graag op dat de productiviteitsverbeteringen voor Spectranetics eerder dan gepland worden gerealiseerd en dat we de Stellarex DCB (Drug Coated Balloon) succesvol in de VS hebben geïntroduceerd. Daarnaast is de integratie van Volcano volgens planning afgerond. Deze activiteit realiseerde een ruime dubbelcijferige vergelijkbare omzetgroei in 2017, met name dankzij de uitstekende prestaties van onze diagnostische katheters, en we hebben de brutomarges verder verbeterd met 10 procentpunten over de afgelopen twee jaar. Ik ben tevreden over de concrete resultaten van de initiatieven die we hebben ontplooid voor autonome groei, zoals de sterk toegenomen orderontvangst van onze activiteit Digital Pathology Solutions, de tweecijferige groei van Sleep & Respiratory Care, en het blijvende succes van Philips OneBlade. Deze revolutionaire hybride styler heeft binnen 18 maanden na de introductie een jaaromzet van meer dan EUR 100 miljoen gegenereerd. We verwachten dat onze markten in 2018 op vergelijkbare basis groeien met 3-5%. Mede dankzij onze goedgevulde orderportefeuille zijn we vol vertrouwen dat we onze middellange-termijndoelen kunnen realiseren van 4-6% groei van de vergelijkbare omzet en een verbetering van de gecorrigeerde EBITA-marge met gemiddeld 100 basispunten per jaar. In het licht van de fasering van onze orderportefeuille verwachten we dat de verbeteringen tegen het einde van het jaar zichtbaar worden." Business segments In the fourth quarter, all business segments continued to deliver operational improvements and increased profitability. In the Diagnosis & Treatment businesses, comparable order intake increased by a strong 12%, driven by North America and China. Comparable sales increased by 6%, reflecting high-single-digit growth in Ultrasound and mid-single-digit growth in Image-Guided Therapy and Diagnostic Imaging. The Adjusted EBITA margin was 90 basis points higher compared to the same period last year, mainly driven by higher volumes, procurement savings and other cost productivity. The 6% comparable sales growth of the Personal Health businesses was driven by high-single-digit growth in Health & Wellness and Sleep & Respiratory Care. The Adjusted EBITA margin improved by 70 basis points, driven by higher volume and procurement savings, partly offset by investments in advertising & promotion. In the Connected Care & Health Informatics businesses, comparable sales increased by 2%, with high-single-digit growth in Healthcare Informatics and low-single-digit growth in Patient Care & Monitoring Solutions. The Adjusted EBITA margin improved by 190 basis points, partly driven by procurement savings and other cost productivity. Comparable order intake showed a low-single-digit decline in the quarter as certain expected large orders were postponed to 2018. Philips’ ongoing focus on innovation through organic and inorganic growth initiatives resulted in the following highlights in the quarter: Cost savings Philips’ productivity programs delivered annual savings of EUR 483 million, ahead of the targeted savings of EUR 400 million. In the quarter, procurement savings amounted to EUR 81 million, led by the DfX program, while other productivity programs generated savings of EUR 52 million. Capital allocation Philips continues to progress with its EUR 1.5 billion share buyback program, which was initiated in the third quarter of 2017 for capital reduction purposes. Details about the transactions to date can be found here. Regulatory update Following the US Food and Drug Administration (FDA) inspection of the Cleveland facility (Illinois) in the third quarter of 2017, Philips submitted its response to the inspectional observations for review by the FDA. In December 2017, the company had a constructive meeting with the FDA. Philips will continue to drive its Quality Management System improvement program, and provide monthly status reports to the FDA highlighting the progress in addressing the observations. On October 31, 2017, a US Federal court formally approved a consent decree that had been agreed to by Philips and the US government, as announced in Philips’ press release on October 11, 2017. Philips is proceeding in line with the terms of the consent decree, which include inspections by independent auditors. As planned, Philips has resumed shipments of its HS1 AEDs globally, as well as consumables, accessories and service parts for all of its defibrillators. Additionally, the company resumed shipments of its FRx and FR3 AEDs to a key market outside of the US in January 2018 and aims to expand these shipments to other markets outside of the US in the remainder of the first quarter of 2018. Philips Lighting As of December 31, 2017, Philips’ shareholding in Philips Lighting was 29.01% of Philips Lighting’s issued share capital. As a result, Philips no longer has control over Philips Lighting and has ceased to consolidate Philips Lighting. The remaining interest in Philips Lighting is presented as an investment included in ‘Assets classified as held for sale’ in the financial statements of Royal Philips as from the end of November 2017. Philips’ net income in the fourth quarter included EUR 562 million related to Philips Lighting’s results in the fourth quarter until the date of deconsolidation and a deconsolidation gain, all of which is reported in Discontinued operations. Philips Lighting will publish results for the fourth quarter and full year 2017 on February 2, 2018. Quarterly Report Fourth Quarter and Annual Results 2017 - Quarterly Report Presentation Fourth Quarter and Annual Results 2017 - Quarterly Results Presentation Conference call and audio webcast A conference call with Frans van Houten, CEO, and Abhijit Bhattacharya, CFO, to discuss the results will start at 10:00AM CET, January 30, 2018. A live audio webcast of the conference call will be available through the link below. Q4 2017 – Fourth quarter and full year 2017 results conference call audio webcast More information about Frans van Houten and Abhijit Bhattacharya Click here for Mr. van Houten's CV Click here for Mr. Bhattacharya's CV
“2017 was een goed jaar, waarin we stappen hebben gezet bij de omvorming van Philips tot marktleider op het gebied van gezondheidstechnologie en onze verbeteringsdoelstellingen voor het jaar hebben gerealiseerd. “Ik ben verheugd over de vergelijkbare omzetgroei van 4%, de verbetering van de gecorrigeerde EBITA-marge met 110 basispunten, en een sterke vrije kasstroom van EUR 1.2 miljard. We hebben onze strategische platforms versterkt met een aantal gerichte acquisities, diverse baanbrekende innovaties geïntroduceerd, meerdere langlopende strategische partnerschappen gewaarborgd en we hebben Philips Lighting gedeconsolideerd als gevolg van het terugbrengen van ons belang tot minder dan 30%.
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a leading health technology company focused on improving people's health and enabling better outcomes across the health continuum from healthy living and prevention, to diagnosis, treatment and home care. Philips leverages advanced technology and deep clinical and consumer insights to deliver integrated solutions. Headquartered in the Netherlands, the company is a leader in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care. Philips' health technology portfolio generated 2017 sales of EUR 17.8 billion and employs approximately 74,000 employees with sales and services in more than 100 countries. News about Philips can be found at www.philips.com/newscenter.
Forward-looking statements and other important information
This document and the related oral presentation, including responses to questions following the presentation, contain certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items. Examples of forward-looking statements include statements made about the strategy, estimates of sales growth, future EBITA, future developments in Philips’ organic business and the completion of acquisitions and divestments. By their nature, these statements involve risk and uncertainty because they relate to future events and circumstances and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these statements. These factors include but are not limited to: global economic and business conditions; developments within the euro zone; the successful implementation of Philips’ strategy and the ability to realize the benefits of this strategy; the ability to develop and market new products; changes in legislation; legal claims; changes in currency exchange rates and interest rates; future changes in tax rates and regulations, including tax reform in the US; pension costs and actuarial assumptions; changes in raw materials prices; changes in employee costs; the ability to identify and complete successful acquisitions, and to integrate those acquisitions into the business, including Spectranetics; the ability to successfully exit certain businesses or restructure the operations; the rate of technological changes; cyber-attacks, breaches of cybersecurity, political, economic and other developments in countries where Philips operates; industry consolidation and competition; and the state of international capital markets as they may affect the timing and nature of the disposal by Philips of its remaining interests in Philips Lighting. As a result, Philips’ actual future results may differ materially from the plans, goals and expectations set forth in such forward-looking statements. For a discussion of factors that could cause future results to differ from such forward-looking statements, see the Risk management chapter included in the Annual Report 2016. Third-party market share data Statements regarding market share, including those regarding Philips’ competitive position, contained in this document are based on outside sources such as research institutes, industry and dealer panels in combination with management estimates. Where information is not yet available to Philips, those statements may also be based on estimates and projections prepared by outside sources or management. Rankings are based on sales unless otherwise stated. Use of non-GAAP information In presenting and discussing the Philips Group financial position, operating results and cash flows, management uses certain non- GAAP financial measures. These non-GAAP financial measures should not be viewed in isolation as alternatives to the equivalent IFRS measures and should be used in conjunction with the most directly comparable IFRS measures. Non-GAAP financial measures do not have standardized meaning under IFRS and therefore may not be comparable to similar measures presented by other issuers. A reconciliation of these non-GAAP measures to the most directly comparable IFRS measures is contained in this document. Further information on non-GAAP measures can be found in the Annual Report 2016. Comparable order intake and Adjusted EBITDA are measures included to enhance comparability with other companies. Use of fair-value measurements In presenting the Philips Group financial position, fair values are used for the measurement of various items in accordance with the applicable accounting standards. These fair values are based on market prices, where available, and are obtained from sources that are deemed to be reliable. Readers are cautioned that these values are subject to changes over time and are only valid at the balance sheet date. When quoted prices or observable market data are not readily available, fair values are estimated using appropriate valuation models and unobservable inputs. Such fair value estimates require management to make significant assumptions with respect to future developments, which are inherently uncertain and may therefore deviate from actual developments. Critical assumptions used are disclosed in the Annual Report 2016 and Semi-Annual Report 2017. Independent valuations may have been obtained to support management’s determination of fair values. Presentation All amounts are in millions of euros unless otherwise stated. Due to rounding, amounts may not add up precisely to totals provided. All reported data is unaudited. Financial reporting is in accordance with the accounting policies as stated in the Annual Report 2016, unless otherwise stated. Market Abuse Regulation This press release contains inside information within the meaning of Article 7(1) of the EU Market Abuse Regulation.
Forward-looking statements
Topics
Media contacts

Ben Zwirs
Philips Global Press Office Tel: +31 6 1521 3446
You are about to visit a Philips global content page
Continue
Steve Klink
Philips Global Press Office Tel: +31 6 10888824
You are about to visit a Philips global content page
ContinueBusiness Highlights Q4 2017
Philips launches new MR solutions designed to support diagnostic confidence, enhance productivity and improve patient experience
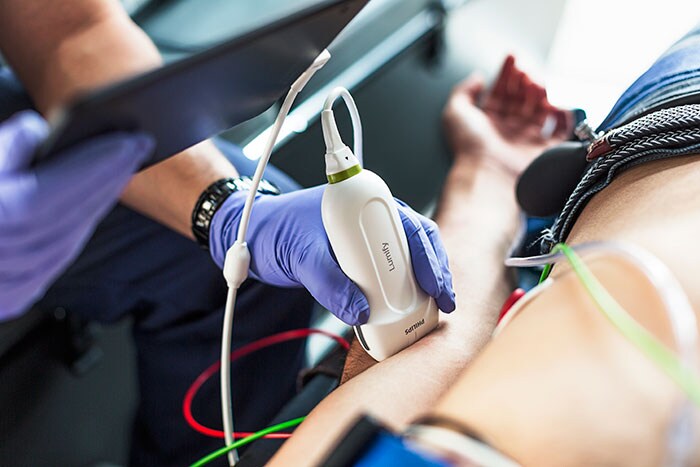
German Armed Forces to use customized Philips Lumify ultrasound for its emergency and rescue operations

Philips and American Well form global partnership in telehealth for consumer health and professional healthcare
Press releases
Get our press releases by e-mail
You are about to visit a Philips global content page
Continue